2023.11.02
细胞外囊泡(EVs)是从细胞中释放出来的纳米级囊泡,具有强大的自分泌和旁分泌生物学活性。EV已被认为构成了一种细胞间通信的基本模式,可在各种情况下介导特定蛋白质、核酸和脂质转运到受体细胞中。因此,EV参与多种疾病的发病机理,包括感染、神经退行性疾病、心血管疾病和癌症等。
外泌体是一种直径约为30-200nm的EV,由于大小低于光学显微镜的衍射极限,直接可视化一直令人望而生畏,因此生理条件下的单颗粒研究也受到阻碍。在本研究中,我们使用英国Oxford Nanoimaging公司研发的随机光学重建显微镜(dSTORM )—Nanoimager对数百个EV进行了3D可视化,根据蛋白标记物定量了亚群,并在单个EV颗粒表面定位了四次跨膜蛋白,为EV的异质性、结构和复杂性提供了新的见解。
数据展示
1. 表达CD81-mCherry和CD63-GFP的细胞产生具有荧光和内吞能力的EV
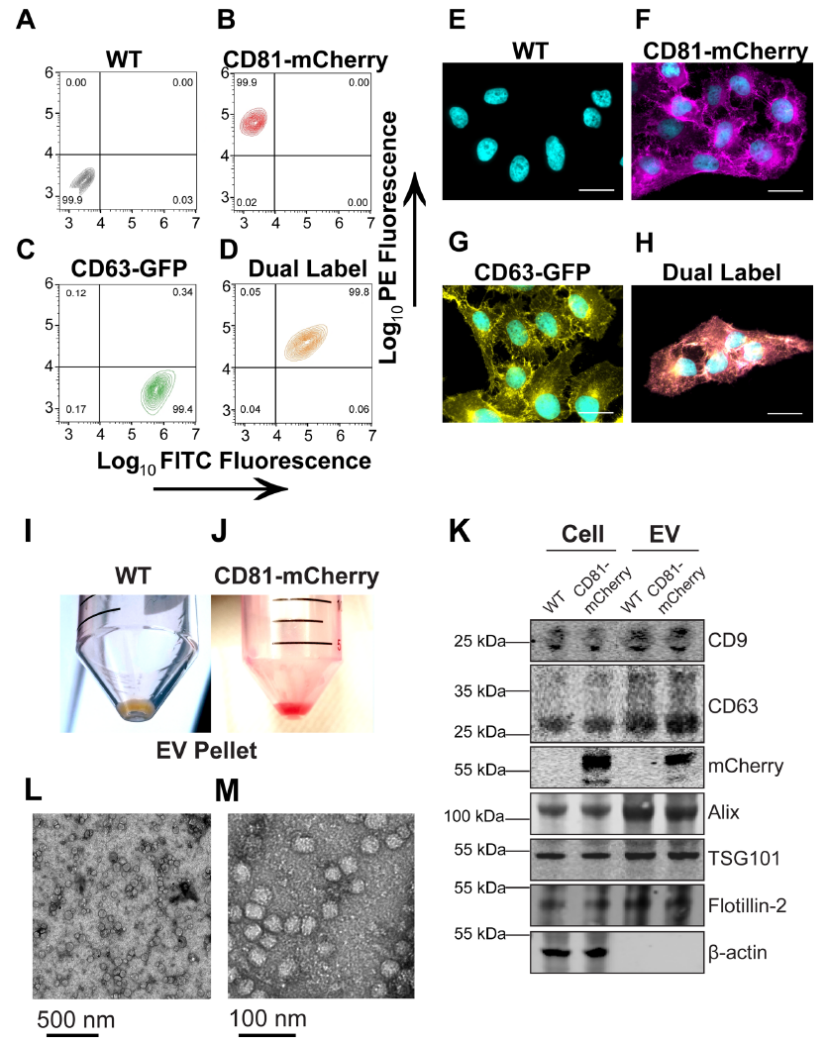
图1.克隆性CD81-mCherry细胞的产生
(A–D) U-2 OS cells were transfected with the indicated plasmid and selected via FACS and cell populations were analysed for clonal expansion via flow cytometry. (E–H) Fluorescence microscopy of cells sorted in A-D (scale bar = 20 μm). (I) EV pellet from U-2 OS WT cells. (J) EV pellet from U-2 OS CD81-mCherry cells. (K) Western blots of cell and EV pellets of U-2 OS WT and CD81-mCherry cells. (L) Transmission EM view of negatively stained affinity-purified CD81+ EVs. (M) Zoomed-in view of a cluster of CD81+ EVs imaged by transmission EM.
四次跨膜蛋白(如CD9、CD63和CD81)是外泌体和其他小EV的常用标记物,可能与ILV货物选择或生物发生有关。在此我们创建了表达绿色荧光蛋白(GFP)标记的CD63或mCherry标记的CD81或两者的克隆U-2 OS细胞系。流式细胞术验证表达稳定(图1A-D),99%以上的细胞表达报告蛋白。与正常加工和运输一致,蛋白质沿着细胞膜和囊泡区室富集(图1E–H)。透射电镜显示出EV预期的球形形态和尺寸(图1L-M)。通过流式细胞术测量(未显示)和共培养测定法(未显示)两种方法测定,CD81-mCherry EVs具有摄取能力。
2. CD63和CD81在细胞同一囊泡内共定位
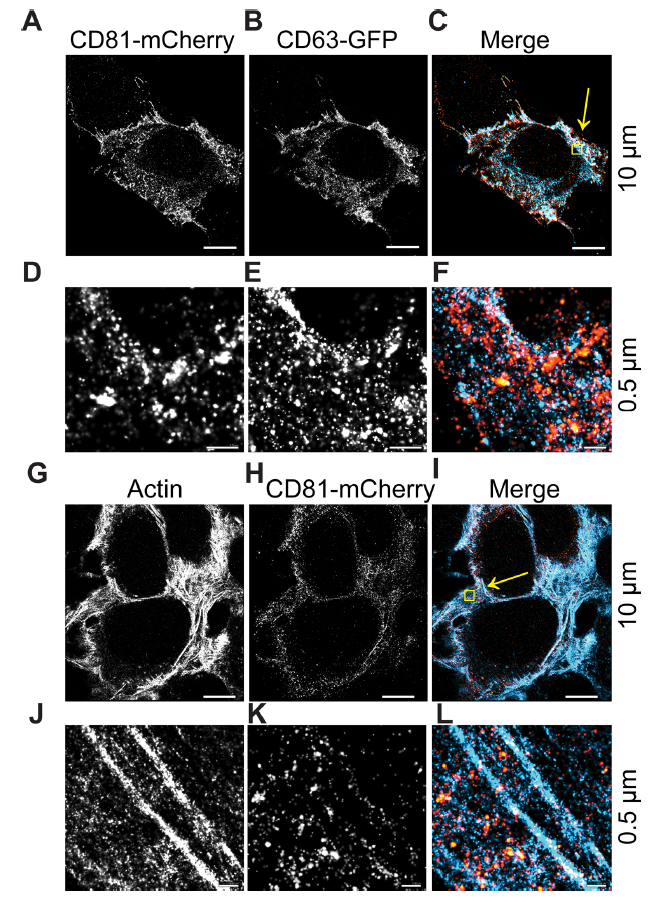
图2.DC81和CD63细胞内共定位区域
(A-C) CD63-GFP and CD81-mCherry U-2 OS expressing cells were visualized by dSTORM. (D–F) Zoomed-in view of yellow box in C. (G–I) CD81-mCherry does not co-occupy regions with β-actin, as visualized by dSTORM. (J–L) Zoomed-in view of yellow box in I. Scale bars are shown with corresponding values on the right for each row of panels
dSTORM系统能够在横向(XY)轴上实现±16 nm的分辨率,在轴向(Z)轴上达到±42 nm的分辨率。因此能够区分单个EV和总EV。在此观察到:10μm范围内,CD63和CD81共定位在细胞内(图2A-C)。在0.5μm范围内,与CD63相比,CD81沿质膜富集,CD63更常见于点状细胞质区域。CD81-mCherry的大球状结构域也很明显,并且同时包含CD63和CD81(图2D–F,黄色)。作为阴性对照,我们使用β-actin,未观察到CD81-mCherry与β-actin的共定位(图2G–L)。
3. 对溶液中单个EV进行dSTORM成像

图3.溶液中单个EV的超分辨率(dSTORM)成像
(A) CD81+ EVs were affinity purified and labelled with the photoswitchable dye CM Red. Max projection image (i.e., pre-dSTORM filtration) is shown. (B) dSTORM filtration of the image in A. (C) Zoomed-in image of a single EV. (D) Frame-index capture of CD81+ EVs in C showing photoswitching throughout the capture. (E) Size distribution analysis of CD81+ EVs viewed through dSTORM using CM Green or CM Red (n = 50 technical replicates, n = 3 biological replicates). (F) Box-Whisker plot of CD81+ EV sizes stained with CM Green or CM Red as measured by dSTORM as compared to NTA
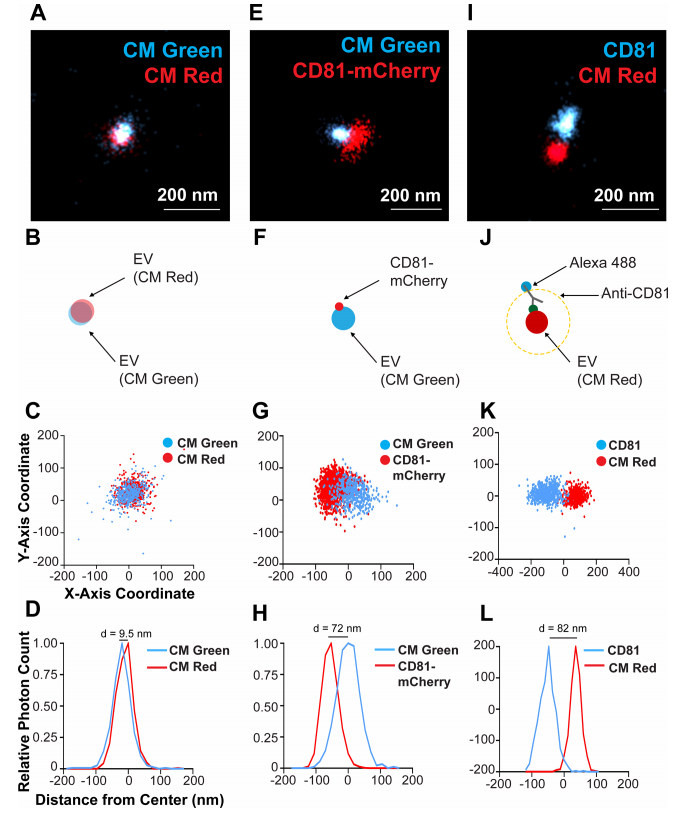
图4.单个CD81+EV的双色染色和定位于膜的CD81的可视化
(A) CD81+ EVs were dual stained with CM Green and CM Red and imaged using dSTORM. (B) Scheme of the experimental setup showing the overlap of membrane dyes. (C) X and Y-axis scatter plot of photoswitching events of a small EV shown in B. (D) Photoswitching event distribution of the EV stained with CM Green and CM Red from the modal centre of the EV in the CM Red channel. The distance of modal centres between the channels is shown. (E) CD81-mCherry EVs were stained with CM Green and imaged using dSTORM. (F) Scheme of the experimental setup showing the offset of the membrane dye with the tetraspanin. (G) X and Y-axis scatter plot of photoswitching events of EV in E. (H) Photoswitching event distribution of the EV in F from the modal centre of the CM Green channel. (I) CD81+ EVs from WT cells were stained with CM Red and the endogenous CD81 was stained indirectly using Alex-fluor antibody conjugates. (J) Scheme of the experimental setup showing the offset of the membrane dye with the antibody spacer directed against the tetraspanin. (K) X and Y-axis scatter plot of photoswitching events of EV in I. (L) Photoswitching event distribution of the EV in I from the modal centre between signals
使用dSTORM对溶液中150个EV成像,生成每种染料的尺寸分布概况(图3A-D),表明EV 尺寸既不是激发/发射波长,也不是染料特异性的,这与NTA分析相当(图3E-F )。因此,dSTORM 构成了一种在生理条件下确定EV大小的新方法。接下来,EV同时用CM Red和CM Green染色(图4A- D)。本实验确定了两种颜色的EV单粒子分析的分辨率极限: 如果两个分子的模态峰彼此直径在d≤10 nm范围内,则认为它们在同一EV上共定位(图4A- D)。CD81-mCherry和CM Green在单个EV上没有共定位(图4E- H)。CD81分子在EV表面的分布并不均匀,像斑块一样集中在 EV 的一个位置,如图4F所示。
4. 单个EV的3D成像
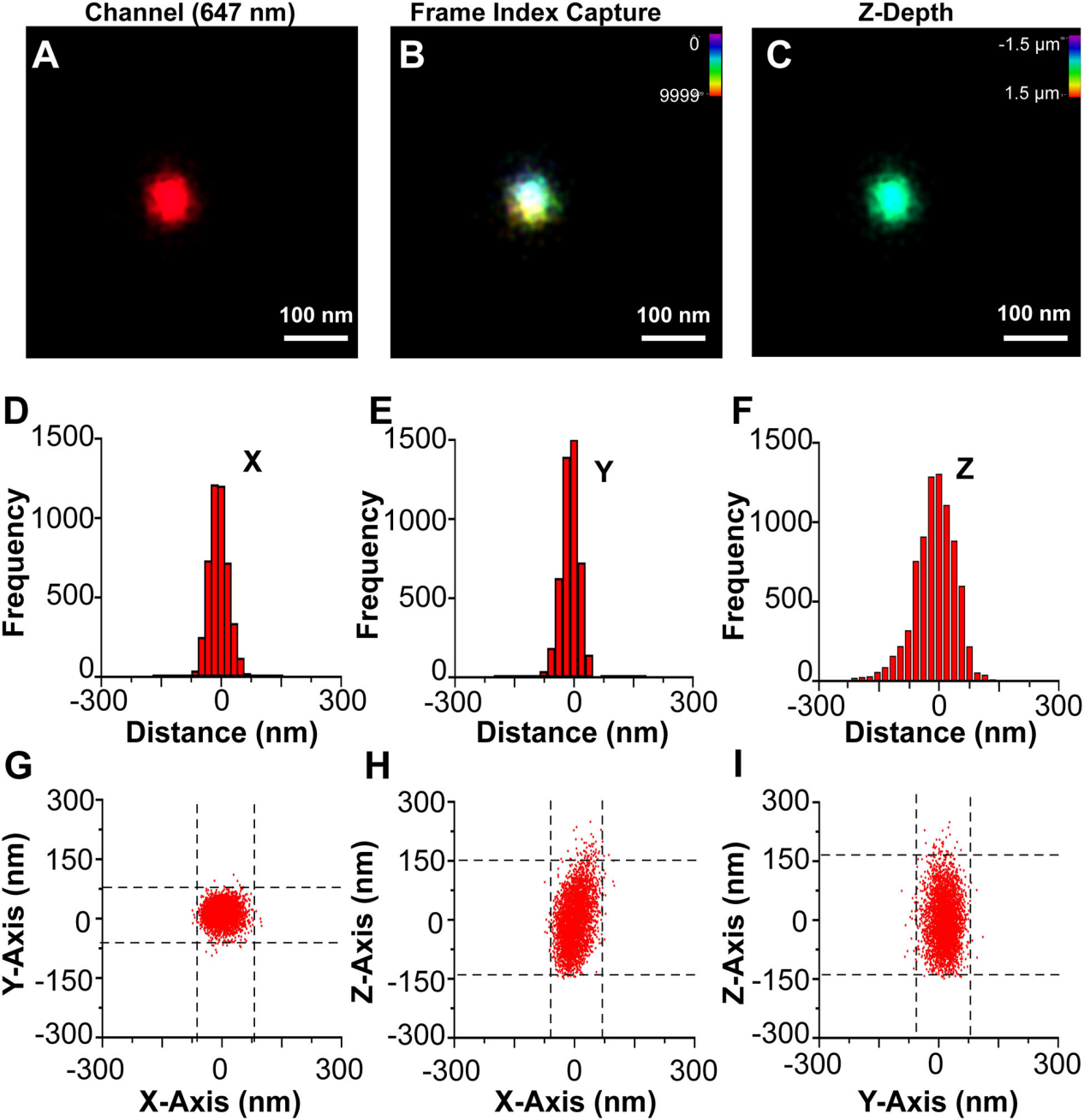
图5.单个CD81+EV的3D dSTORM
(A) A single CD81+ EV fromWT cells stained with CM Red was visualized by dSTORM with Z-axis astigmatism activated. (B) Frame index capture of the CD81+ EV shown in A. (C) Z-depth information of the CD81+ EV in A. (D) X-axis size histogram of EV with events plotted from the modal centre. (E) Y-axis size histogram of EV with events plotted from the modal centre. (F) Z-axis size histogram of EV with events plotted from the modal centre. (G) X and Y-axis scatter plot of photoswitching events of an EV. (H) Same as G, but for the X-Z axis. (I) Same as G, but for the Y-Z axis
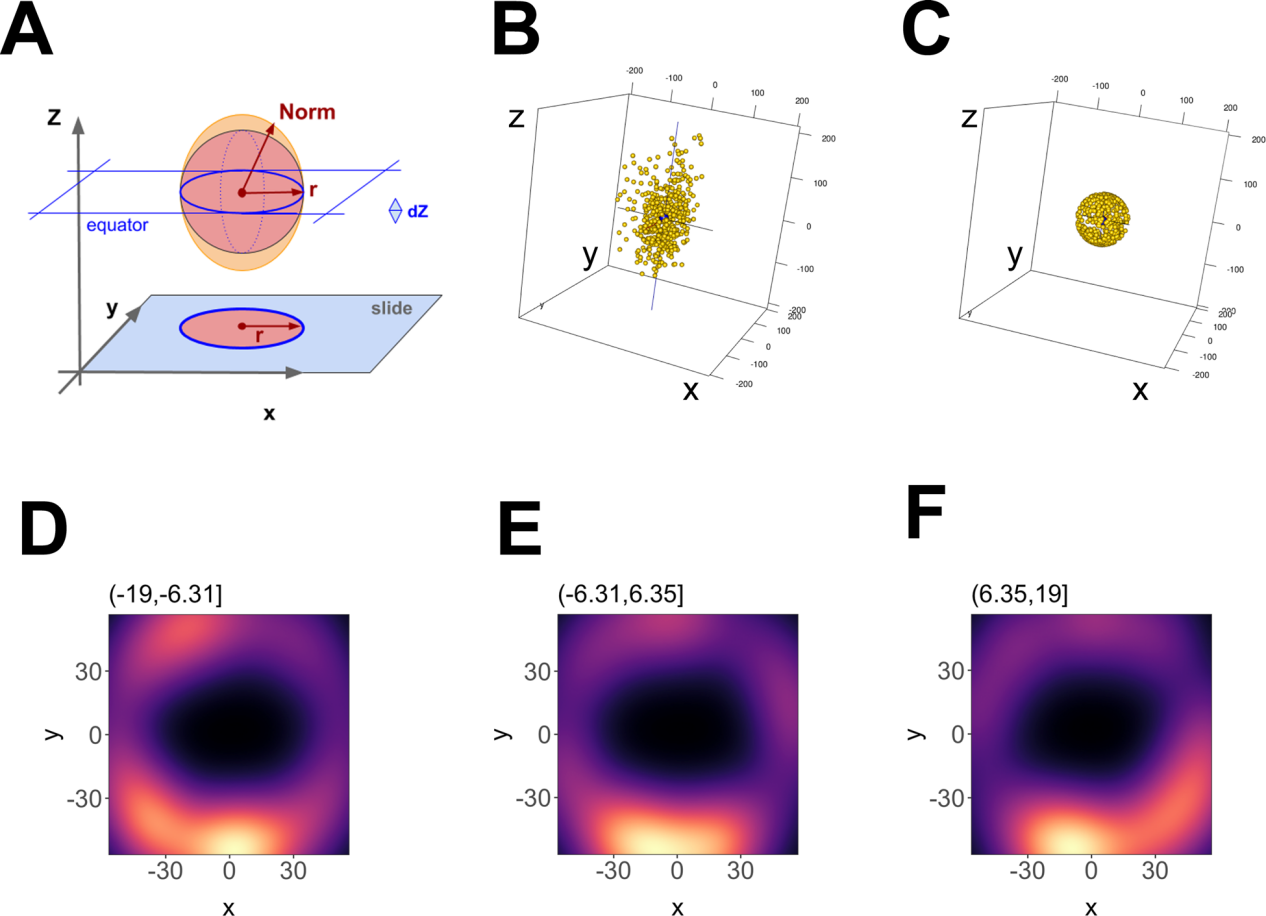 图6.单个EV的三维重建
图6.单个EV的三维重建(A) Outline of the geometric foundation, with “slide” indicating the focal plane at z = -max. The filled red circle and red radius depict how a 2D image would look like. Above, the red sphere indicates the ideal EV, and the orange ellipsoid indicates the actual data. The red arrow r = sqrt (x2 + y2) depicts the radius at the equator, whereas “Norm” indicates the point vector from the centre to any point on the ellipsoid surface.Norm = sqrt (x2 + y2 +z ), which at z = 0 equals r. (B) 3-D representation of the data before transformation and (C) after transformation. (D) Principal component analysis (PCA) plot of an EV using 12 nm Z-axis binning of photoswitching events, showing hollowed core. (E) Same as D, but for a bin at one Z-axis increment shift. (F) Same as E, but for a bin at one Z-axis increment shift
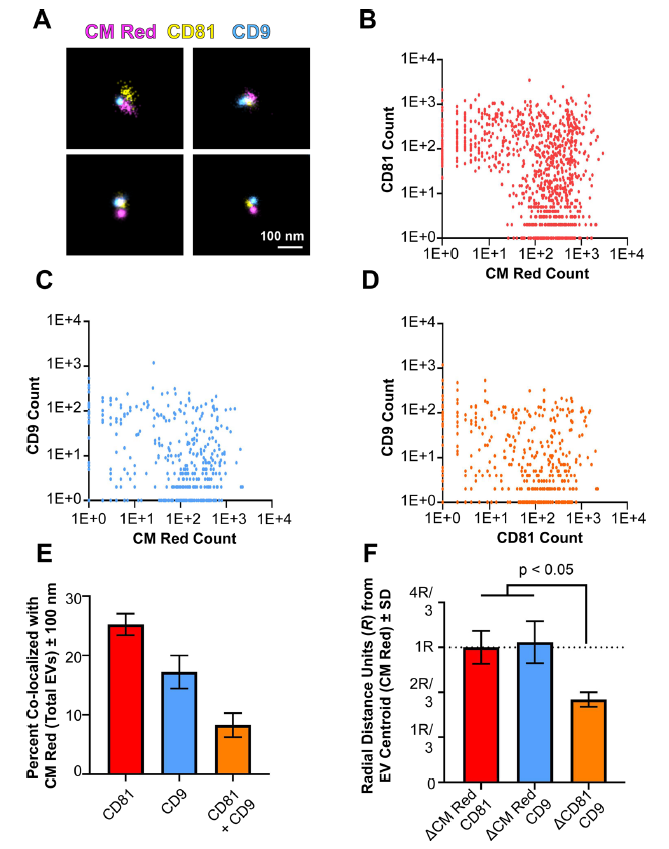
图7.单个EV表面的四次跨膜蛋白簇
(A) Three-colour dSTORM was performed on total EVs using emissions from CM Red,CD81-mCherry, and anti-CD9 Alexafluor-488. Four representative images are shown: scale = 100 nm. (B) Clustering of photoswitching events of total EVs that were positive for CMRed and CD81-mCherry. X- and Y-axis show events collected post-super-resolution filtration in log scale. The max radius between modal centroids allowed was 150 nm (C) Same as B, but for co-localizing events for CM Red and CD9. (D) Same as B, but for co-localizing events for CD81-mCherry and CD9. (E) The total number of EVs per exposure was quantified using the non-specific membrane-intercalating dye CM Red and co-localizing frequencies were determined for CD81, CD9, or both. (F) The distance between modal centroids was determined between CM Red and CD81, and set to 1 radial measurement, R (dotted line). This was then compared to the distance between the centre of an EV and CD9, as well as between CD81 and CD9. For all experiments, three independent exposures were taken with > 900 individual EVs identified through CM Red clusters
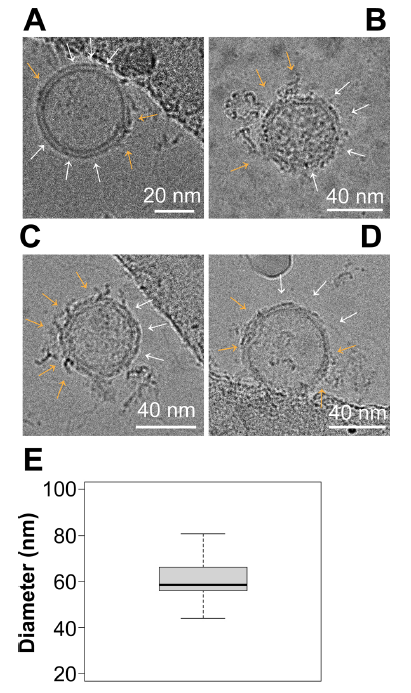
图8.Cryo-EM显示EV表面有富含蛋白质的簇
(A–D) Representative images of EVs viewed under Cryo-EM. Surface nanodomains are shown with an orange arrow, highlighting the protein and/or lipid-dense regions of an individual EV. Empty regions are shown with white arrows. (E) Size analysis of EVs viewed through Cryo-EM. EVs were measured in diameter in nanometers based on their lipid bilayer and do not include protruding proteins from the surface
在多个轴向(Z)深度处收集光开关事件(图5A- C)。3D dSTORM与传统显微镜一样,沿z轴的分辨率小于沿XY轴的分辨率(图5D-F ),使用dSTORM在三维和多色通道中完成对单EV重建,这种直接可视化可用于确定 EV 表面上结构域和蛋白质复合物的存在,如图6所示。
为了进一步提供局部结构域的证据,对单个EV表面的两种不同的四次跨膜蛋白进行了3D成像,CMRed和CD 81- mCh erry 阳性的EV被鉴定为双阳性(图7B)。CD9也与CM Red 共定位,但出现在较少的EV上(图7C)。最后,仅对 CM Red 阳性的信号绘制两种四次跨膜蛋白的EV信号(图7D)。该方法鉴定了仅携带CD81的EV、仅携带CD9的EV和同时携带CD81和CD9的EV。异位过表达的CD81- mCherry在总EV中的定位水平(25.26 %±2.58 )高于CD9(17 .23 %± 3.94 )(图7E)。
冷冻电镜(Cryo-EM)可以获得分子结构的埃级(Å)细节,尤其是对于病毒这样较大的生物实体:EV平均直径为 60.3±10.5 nm (mean±sd, 95 % CI = 51.75 - 68.93,n=9个独立图像/每组,3组生物学重复)(图 8E),大小符合预期。根据EV的方向和截面,在单个EV上可以看到多个域(图 8A-D )。黄色箭头表示蛋白质和脂质密集区域,白色箭头表示没有蛋白质或脂质密集斑块的区域。冷冻电镜(Cryo-EM)结果与3-D dSTORM结果一致,证明了EV表面存在不同的微结构域。
结果讨论
作为 3d dSTORM在EV上的应用,本研究旨在揭示和记录EV的结构异质性,并研究EV 膜微区。结构表面组织和组成复杂性是EV和人工脂质体之间的关键区别,人工脂质体只包含有限数量的不同脂质,不含蛋白质。通过dSTORM可以识别单个和不同尺寸EV表面的四跨膜蛋白。这点得到了Cryo-EM的验证。总之,我们的研究表明,EV包含以前未被识别的表面结构和空间组织。对EV表面组装的进一步研究可能会对其细胞内组装、特定包装和组织特异性目的地产生新的见解。
dSTORM Training Kit
近日,Oxford Nanoimaging新开发的dSTORM Training Kit已发布 。使用此试剂盒,您不仅可以体验简洁方便的超分辨显微成像工作流程,还可以学习单分子定位显微镜的基本原理:
了解dSTORM成像的样品制备
用合适的试剂为dSTORM制备样品
了解dSTORM成像的基础知识
在Nanoimager上获取dSTORM数据
在试验自己的样品前获取自信
分析CODI上的dSTORM数据
如果您想更多了解,请联系我们。




